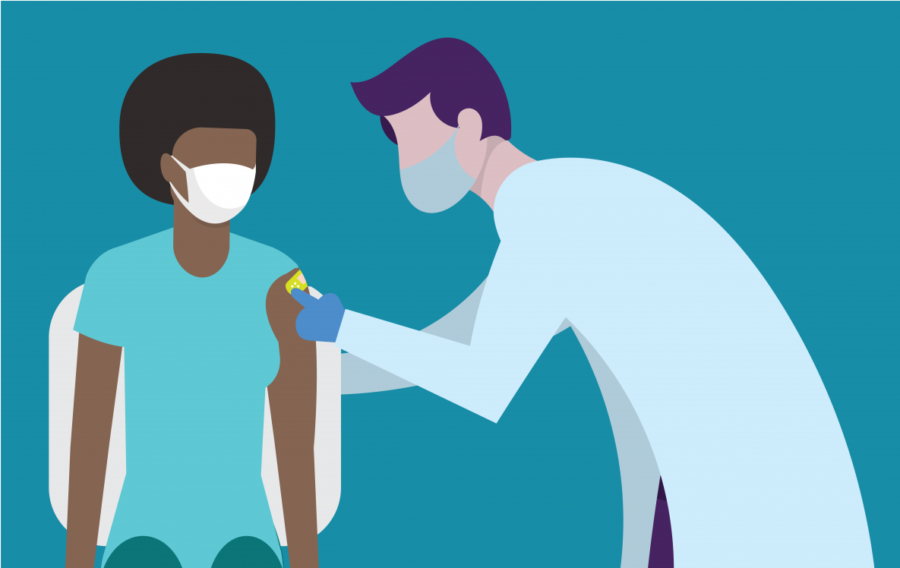
U.S. Resumes Use of Johnson & Johnson Vaccine
UPDATE: On April 21, Europe health officials decided to resume the distribution of the Johnson & Johnson vaccine. On April 24, the CDC resumed use of the vaccine.
The saga isn’t over yet for Johnson & Johnson’s Covid vaccine.
The Food and Drug Administration (FDA) and Centers for Disease Control (CDC) decided on April 13 to pause the use of the Johnson & Johnson one-shot vaccine after six women developed rare brain blood clots. Experts link these blood clots similar to Europe’s AstraZeneca vaccine, which is not yet approved in the United States.
According to ABC News roughly seven million people have received the shot. The other vaccines are made by Moderna, and Pfizer; 187.5 million people in the United States have received at least the first dose of their vaccine, according to Fortune.
According to the New York Times, six women between the ages 18 and 48 have been affected by rare blood clots attributed to the Johnson & Johnson vaccine; one woman has died while there is another hospitalized in critical condition.
Dr. William Schaffner, an infectious disease physician at Vanderbilt University with expertise in preventive medicine and health policy, said in the ABC News article that both vaccines are made in a similar way, “but the carriers are different kinds of adenoviruses… that’s part of the background information, why indeed there is a pause now.”
On April 21, the European Union reversed it’s decision and began using the Johnson & Johnson vaccine. In a press release, the EU said: “the Johnson & Johnson vaccine should carry a warning for a potential risk for rare blood clots, though the agency said it believes the shot’s benefits outweigh risks.”
Nathan Shatz got the Johnson & Johnson vaccine April 1 in Deerfield.
“I was extremely relieved to get the vaccine because I had been waiting specially for a J and J dose for a while,” said Nathan, a day student from Northampton. “I’ve been eligible since February but couldn’t get a J and J appointment until April.”
Nathan in an email expressed his frustrations about the pause of the vaccine.
“When I heard the news that J and J rollout was being paused I became frustrated, not because I was worried for my own health but because I am aware of the implications of the pause,” he mentioned. “Specifically I am frustrated by the fact that the six documented cases of blood clots from millions became the central focus of the media for a number of days, which increased anti-vax sentiment.”
He is more worried about people doubting the efficacy and legitimacy of vaccines.
“I’m not worried about my own safety,” he added. “I trust Johnson & Johnson and the bureaucratic process involved in the vaccine rollout. I think it’s foolish to assign sudden distrust to the J and J vaccine specifically because it is an adenovirus variant which we have had success with in the past. Also, the pause prevents many Americans in lower-income areas from receiving the single-dose solution they need.”
Carrie St. Marie, an 18-year old senior day student from Longmeadow, Mass., got her Johnson & Johnson Covid vaccine on April 8 in Springfield, Mass.
“At first I felt fine, and then flu like symptoms hit me hard for the next 48 hours. I had a high fever, chills, and body aches, It wasn’t fun but definitely was worth it.”
With slim chances of getting blood clots, Carrie was not concerned.
“When the news came out that weekend that they paused that vaccine I was a little nervous but the chances of something like that happening is very slim so I’ve haven’t been worried about it as much,” she said.
In a press release issued April 20, Johnson & Johnson explained: “The health authorities advise that people who have received our COVID-19 vaccine and develop severe headache, abdominal pain, leg pain, or shortness of breath within three weeks after vaccination should contact their health care provider.”
Johnson & Johnson is a company known for the research, development, and manufacturing in the healthcare field. They are primarily known for their products related to human health and well-being.